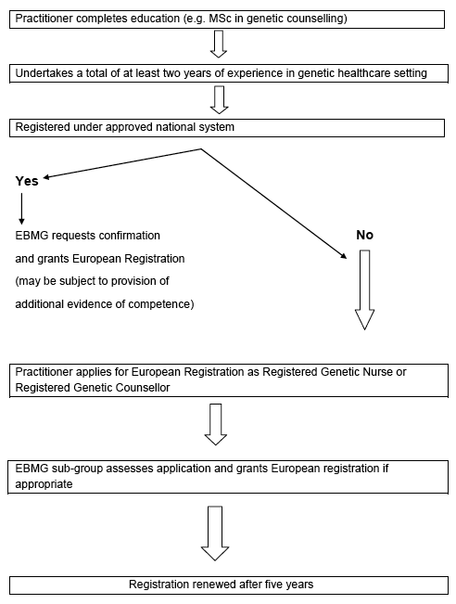
Section 7. Maintenance of registration
Registration will be granted for a period of five years. Registrants will then have to renew registration through submission of a record of continuing professional development (CPD) and two references, one from a senior colleague and one from the line manager. The referees must confirm that the registrant is still working in a role directly relevant to the profession and works within the Code of Professional Practice. Please see the European Registration Renewal section.
Registrants must record the date and type of education, but also write reflective comments on what they learnt from the education and how they have integrated that into their practice. Demonstration of integration of the learning into practice is an essential part of the CPD record. For a series of lectures or seminars, please list the actual dates and topics.
Continuing professional development will be recorded on Form F and should be signed by the departmental manager.